Specific Heat of Silver J/g C
What is the total amount of heat needed to change 2250 g of silver at 00C to 2000C. The specific heat of silver is 0234 jgc.

What Is The Specific Heat Of Silver In 2021 Heat Silver 10 Things
Specific heat of Silver is 0235 Jg K.

. Calculate the mass of the sample ofsilver g. Now look at your periodic table and choose a metal that is most likely the identity of the sample. Answer H ms delta t or s H m delta t 46810 249 130 Jg degree C 048 Jg degree c No.
Of moles in 810 g Ag 810 g1079 gmol 0075 mol H 0075 mol 2533 Jmol degree c 188 degree C 357 J specific heat Molar heat capacityAtomic mass 2535 Jmol degree c1079 Jg degree C. Calculate the change in energy of the gas. The intensive properties c v and c p are defined for pure simple compressible substances as partial derivatives of the internal energy uT v and enthalpy hT p respectively.
31 rows Substance Formula Phase C sp Jg o C. We will assume m 5 kg. C It takes 115 kJ of energy toheat a sample of pure silver from 120C to 149 C.
Which coin requires more heat to raise its temperature by 40C. Jg C Transcribed Image Text. The heat capacity of freshwater is 4182 Jg K and the heat capacity of salt water is 3993 Jg K.
The specific heat of silver is 0129 JgC 2. The work done to compress a gas is 670 J. An unknown metal has a mass of 180 g.
As a result 290 J of heat is given off to the surroundings. A piece of silver with a mass 348 g has a heat capacity of 825 JC. Specific heat or specific heat capacity is a property related to internal energy that is very important in thermodynamics.
The specific heat of silver is 024 JgC. QsmDeltaT where q heat absorbed or lost s is the specific heat capacity m is the mass and DeltaT is the change in temperature final temperature-initial temperature From what the problem gave us we can plug in the numbers. What is the specific heat of silver.
How many joules of energy are needed to warm 737 g of silver from 250C to 275C. The specific heat of silver is 024 JgC. If 1357 J of heat are added to 540 g of water initially at 250 C.
The final temperature of the two blocks will be. What mass of silver will absorb 4200 J of heat when heated from 243 C to 852 C. 71 rows The specific heat is the amount of heat energy per unit mass required to raise the.
Material JkgK BtulbmF JkgC kJkgK Aluminium 887 0212 887 0887 Asphalt 915 021854 915 0915 Bone 440 0105 440 044 Boron 1106 0264 1106 1106 Brass 920. If 154 g of silver absorb 332 J heat how much will the temperature of the silver increase. Silver has a specific heat capacity of 024 J gC Round your answer to the nearest whole number and units of g are assumed so dont type them.
If the temperature of the metal sample rises from 150C to 400C as the sample absorbs 890 J of heat what is the specific heat of the sample. The higher the heat capacity the more slowly the water will heat given the same amount of energy added. Up to 24 cash back Specific Heat Practice Problem 1 1.
Is caused by particles bumping into each other faster and then pushing out on the containerThermal expansion is simply the increase of an object volume due to an increase in temperature. What is the specific heat of silver. Determine the mass of the sample.
Chemistry questions and answers. The equation used to solve for specific heat capacity is this. The specific heat capacity of silver is 024 JC g.
Solution show work including units. Up to 256 cash back The specific heat capacity of silver is 024 Jg C. How many kilojoules are required to raise the temperature of a 150.
Calculate specific heat as c Q mΔT. You can also go to advanced mode to type the initial and final values of temperature manually. The specific heat capacity of silver is 024 JCg.
What is the total amount of heat needed to change 2250 g of silver at 2000C to 00C. Then ΔT -3 K. 890 J180 g x 250C specific heat 0199 J.
Silver chloride often used in silver plating contains 7527 AgCalculate the mass of silver chloride required to plate 185 mg of pure silver. Does salt water have a higher specific heat capacity. Suppose a 500 g block of silver specific heat 02350 JgC at 100C is placed in contact with a 500 g block of iron specific heat 04494 JgC at 000C and the two blocks are insulated from the rest of the universe.
This is the best answer based on feedback and ratings. The specific heat capacity of materials ranging from Water to Uranium has been listed below in alphabetical order. In our example it will be equal to c -63000 J 5 kg -3 K 4200 J kgK.
Therefore saltwater will heat up faster than freshwater. Below this table is an image version for offline viewing. Calculate the energy required to raise the temperature of 1500 g Ag from 273 K to 298 K.
G sample of silver from 25 C to 135 C. 473s825 Divide by 825 on both sides. The specific heat of silver is 0129 JgC 58050 J-58050 J.
Silver has a specific heat of 0235 Jg C. T m c 12. The specific heat of silver is 024 JgC.
Calculate the energy required to raise the temperature of 1540 g Ag from 274 K to 298 K. Calculate the energy required to raise the temperature of 10 mole Ag by 10 C. The specific heat of silver is 0235 JgC and the specific heat of gold is 0130 JgC.
Calculate the amount of energy that is needed to raise the temperature of 175 g of silver from 225c to 400c. Energy J Calculate the energy required to raise the temperature of 10 mole of Ag by 10C called the molar heat capacity of silver. Chem The specific heat capacity of.
Heat Capacity Gases Definition Calculation Units Formula
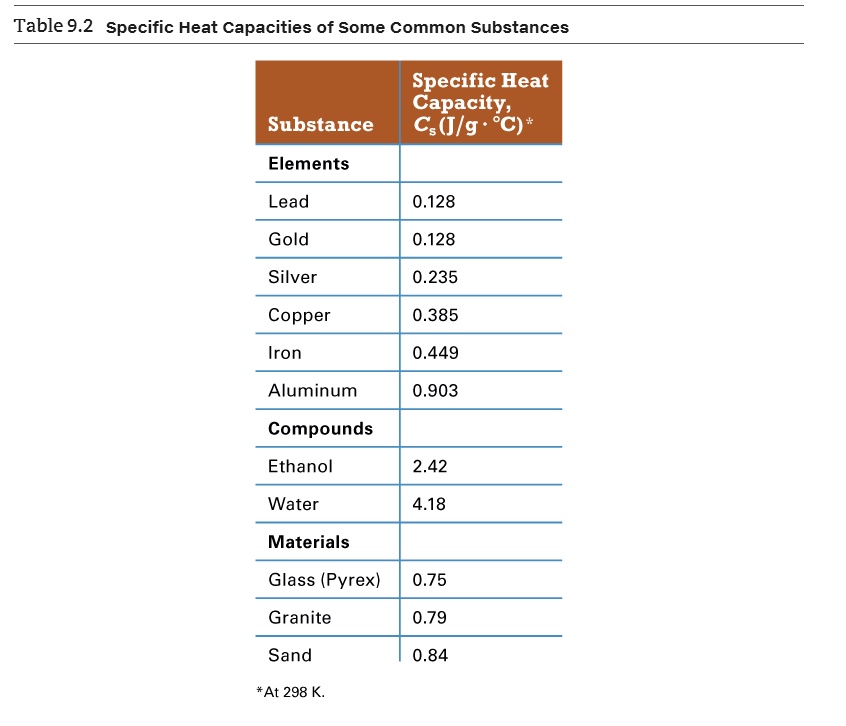
Solved Table 9 2 Specific Heat Capacities Of Some Common Substances Specific Heat Capacity Cs Jlg C Substance Elements Lead 0 128 Gold 0 128 Silver 0 235 Copper 0 385 Iron 0 449 Aluminum 0 903 Compounds Ethanol 2 42 Water

Specific Heat Capacity Of Materials The Engineering Mindset

Specific Heat Of A Metal By Calorimetry Youtube

Can Anyone Suggest A Material With The Highest Specific Heat Capacity Higher Than Water

Specific Heat Heat Of Fusion And Vaporization Example Video Khan Academy
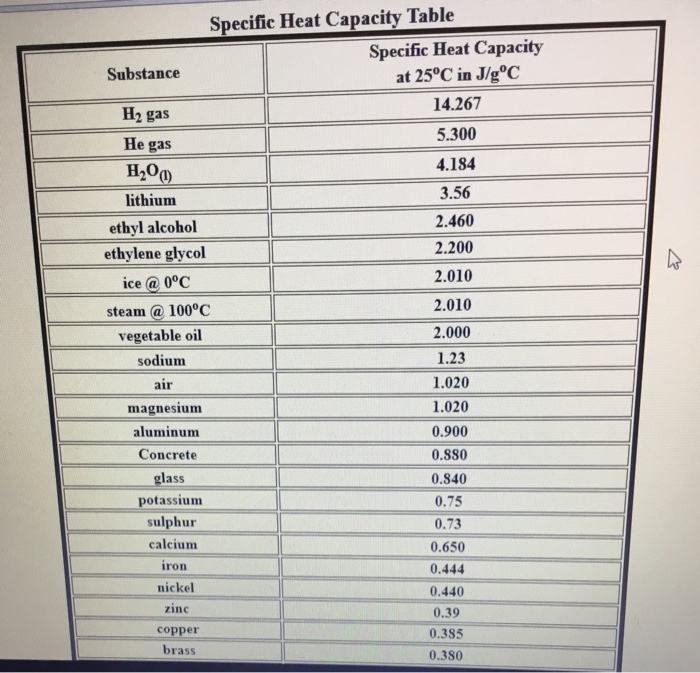
Solved Activity 6 Practice 2 1 Considering That The Chegg Com
Solved To Determine The Specific Heat Of An Object A Chegg Com
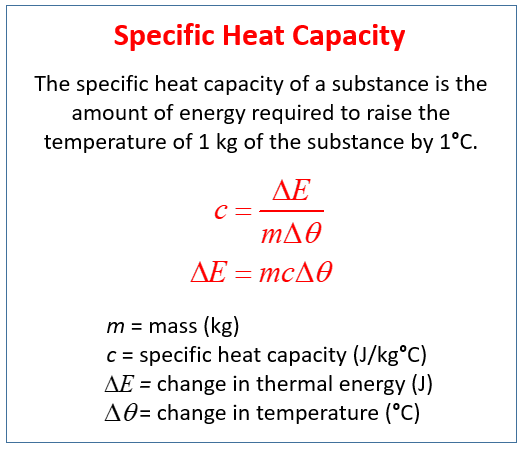
Specific Heat Capacity Video Lessons Examples Step By Step Solutions
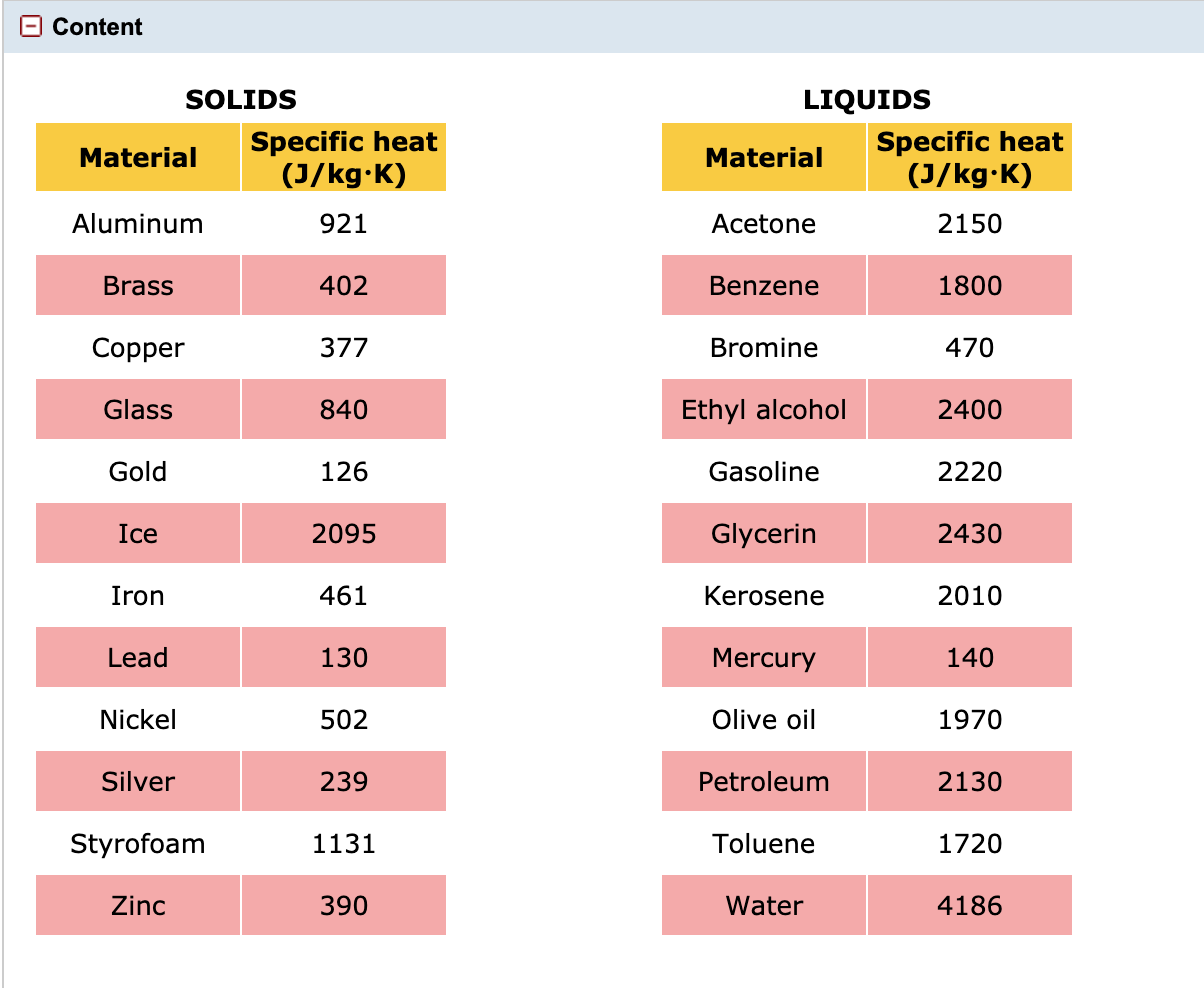
Solved O Content Solids Specific Heat Material J Kg K Chegg Com
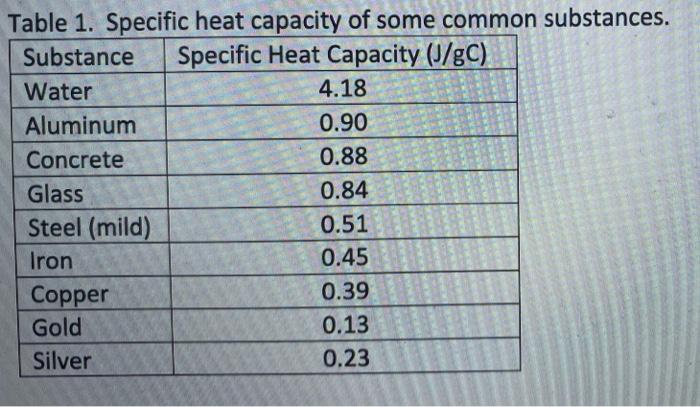
Solved Table 1 Specific Heat Capacity Of Some Common Chegg Com
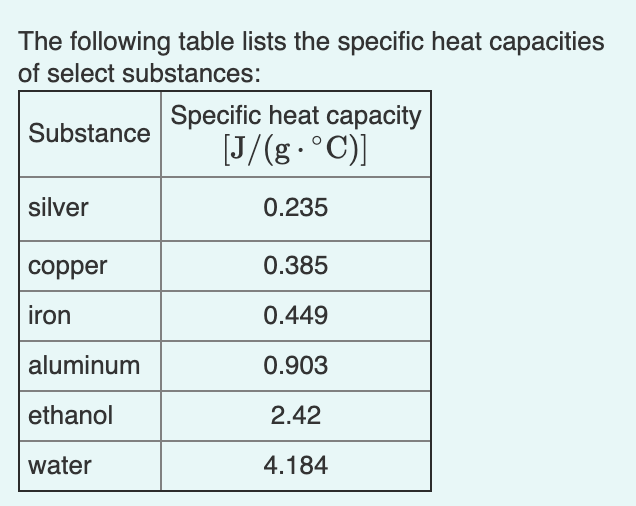
Solved The Following Table Lists The Specific Heat Chegg Com

Specific Heat Definition Facts Britannica
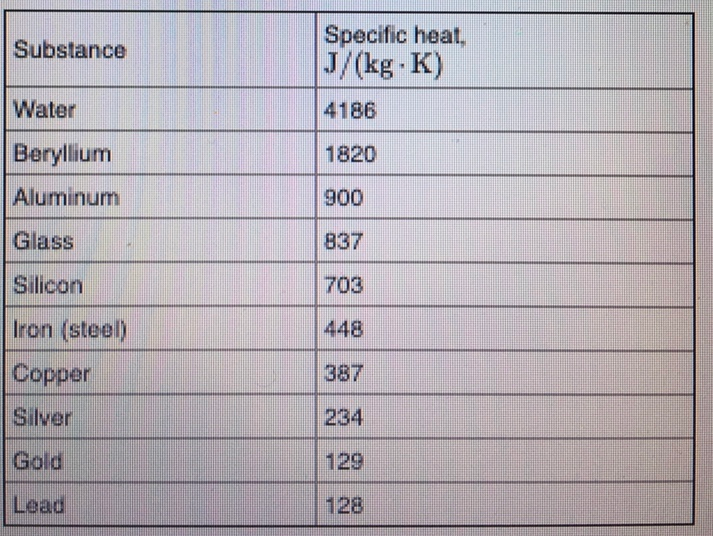
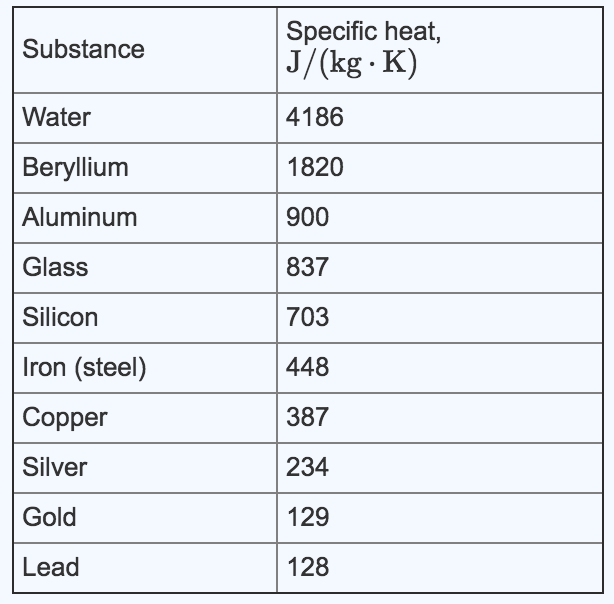
Solved Substance Water Specific Heat J Kg K 4186 1820 Chegg Com
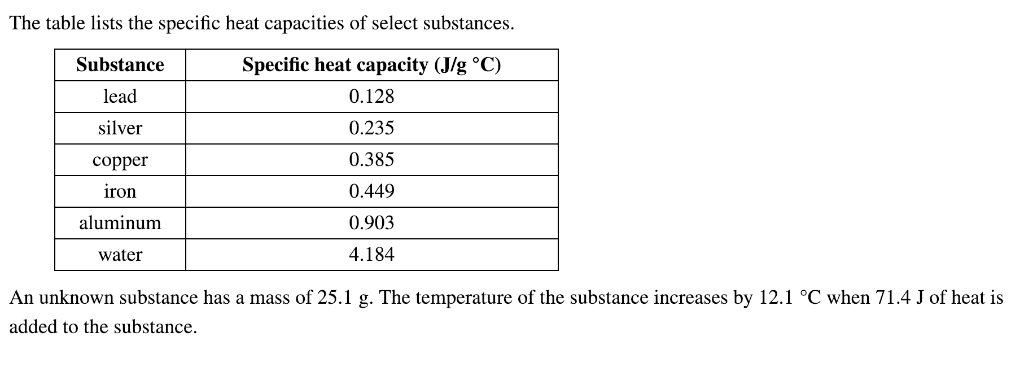
Solved The Table Lists The Specific Heat Capacities Of Chegg Com
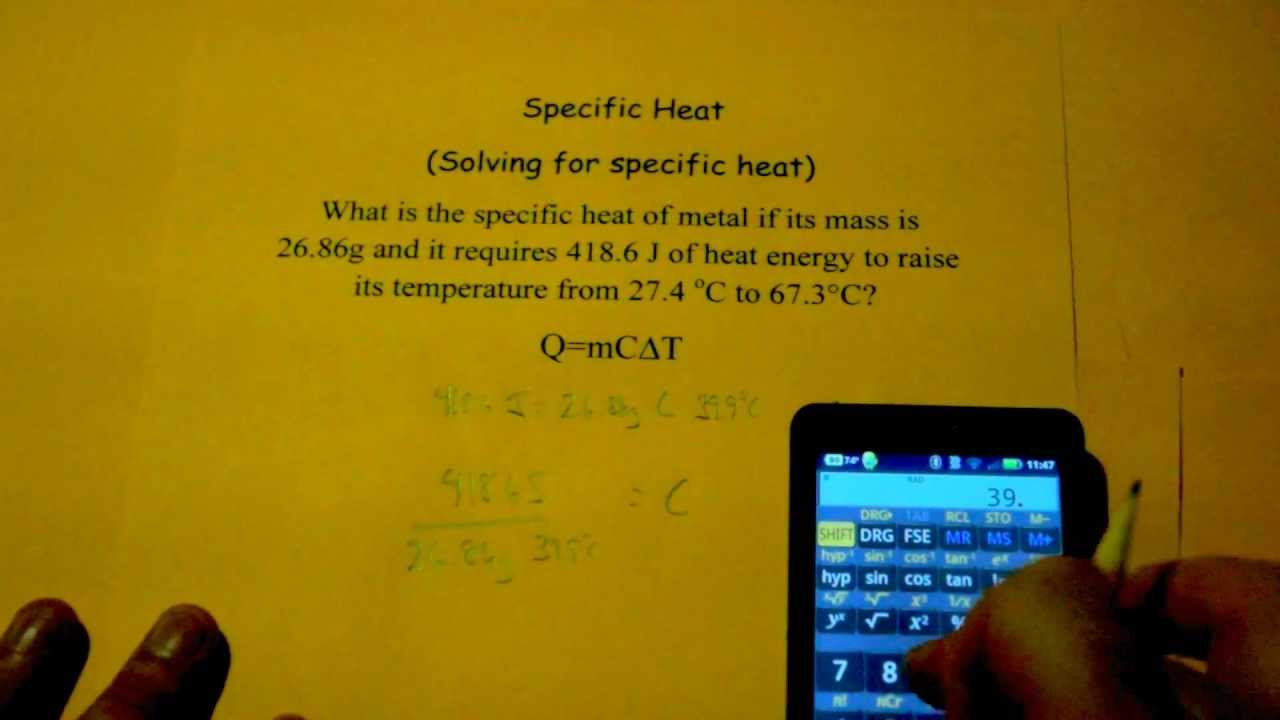


Comments
Post a Comment